Refractory Metal Technical Data
Refractory Metals: Typical Analysis
| Element | Maximum % Molybdenum | Maximum % Tungsten | Maximum % Tantalum | Maximum % Niobium |
|---|---|---|---|---|
| Aluminum | 0.001 | 0.002 | --- | 0.005 |
| Calcium | 0.003 | 0.003 | --- | --- |
| Chromium | 0.005 | 0.002 | --- | --- |
| Copper | 0.001 | 0.002 | --- | --- |
| Iron | 0.005 | 0.003 | 0.010 | 0.01 |
| Lead | 0.002 | 0.002 | --- | --- |
| Magnesium | 0.001 | 0.002 | --- | --- |
| Molybdenum | 99.95 Min | --- | 0.010 | 0.01 |
| Manganese | 0.001 | 0.002 | --- | --- |
| Nickel | 0.001 | 0.003 | 0.005 | 0.005 |
| Silicon | 0.003 | 0.002 | 0.005 | 0.005 |
| Tin | 0.003 | 0.002 | --- | --- |
| Titanium | 0.002 | 0.002 | 0.005 | --- |
| Tantalum | --- | --- | 99.90 Min | 0.2 |
| Tungsten | --- | 99.95 Min | 0.030 | 0.05 |
| Carbon | 0.005 | 0.005 | 0.0075 | 0.01 |
| Oxygen | --- | --- | 0.020 | 0.025 |
| Nitrogen | --- | --- | 0.0075 | 0.01 |
| Hydrogen | --- | --- | 0.0001 | 0.0015 |
| Niobium | --- | --- | 0.050 | 99.9 |
Typical Properties of Molybdenum, Tantalum, Tungsten
Ranges only: Data will vary with type of sample and previous work history
| Molybdenum | Tungsten | Tantalum | ||
|---|---|---|---|---|
| Property | Atomic Number | 42 | 74 | 73 |
| Atomic Weight | 95.95 | 183.86 | 180.95 | |
| Atomic Volume | 9.41 | 9.53 | 10.90 | |
| Lattice Type | Body centered cube | Body centered cube | Body centered cube | |
| Lattice Constant; 20°C, A |
3.1468 | 3.1585 | 3.3026 | |
| Isotope (Natural) | 92, 94, 95, 96, 97, 98, 100 | 180, 182, 183, 184 186 | 181 | |
| Mass | Density at 20° C gm/cc | 10.2 | 19.3 | 16.6 |
| Density at 20° C lb/in 3 | 0.368 | 0.697 | 0.600 | |
| Thermal Properties | Melting Point, °C | 2610 | 3410 | 2996 |
| Boiling Point, °C | 5560 | 5900 | 6100 | |
| Linear Coefficient of Expansion per °C | 4.9 x 10-6 | 4.3 x 10-6 | 6.5 x 10-6 | |
| Thermal Conductivity at 20°C, cal/cm2/cm°C/sec. | 0.35 | 0.40 | 0.130 | |
| Specific Heat, cal/g/°C; 20°C | 0.061 | 0.032 | 0.036 | |
| Electrical Properties | Conductivity, % IACS | 30% | 31% | 13% |
| Resistivity, microhms-cm; 20°C | 5.7 | 5.5 | 13.5 | |
| Temperature Coefficient of Resistivity per °C (0-100°C) | 0.0046 | 0.0046 | 0.0038 | |
| Mechanical Properties | Tensile Strength at room temperature, psi | 100,000-200,000 | 100,000-500,000 | 35,000-70,000 |
| Tensile Strength-500°C psi | 35,000-65,000 | 75,000-200,000 | 25,000-45,000 | |
| Tensile Strength-1000°C psi | 20,000-30,000 | 50,000-75,000 | 13,000-17,000 | |
| Young's Modulus of Elasticity; lb/in2 | ||||
| Room Temperature | 46 x 106 | 59 x 106 | 27 x 106 | |
| 500°C | 41 x 106 | 55 x 106 | 25 x 106 | |
| 1000°C | 39 x 106 | 50 x 106 | 22 x 106 | |
| Spectral Emissivity | (Wave Length approx. 0.65) | 0.37 (1000°C) | 0.45 (900°C) | 0.46 (900°C) |
| Working Temperature | 1600°C | 1700°C | Room | |
| Recrystallizing Temp | 900-1200°C | 1200-1400°C | 1000-1250°C | |
| Stress Relieving Temp | 800°C | 1100°C | 850°C | |
| Metallography | Etchant | Hot H2O2; 6% sol | HF-NH; F sol | Alk.K3FE(CN) sol |
| Polishing | Alumina - Rouge to finish | |||
Note: Etch and polish repeatedly until grain boundaries appear.
Data on Molybdenum
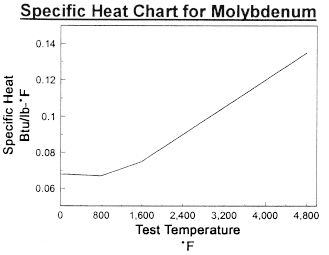
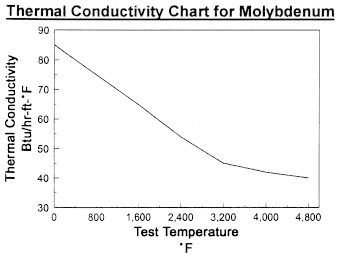
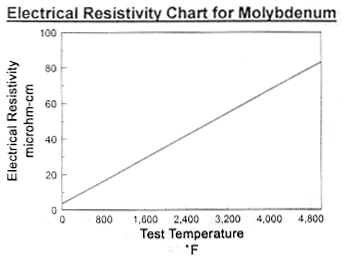
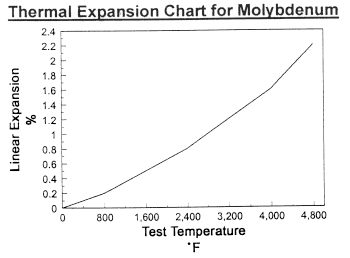
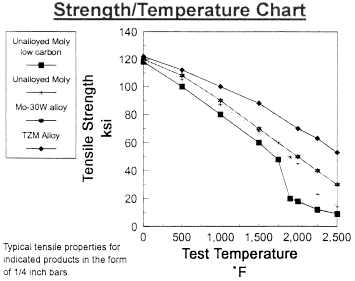
Data on Molybdenum and Tungsten
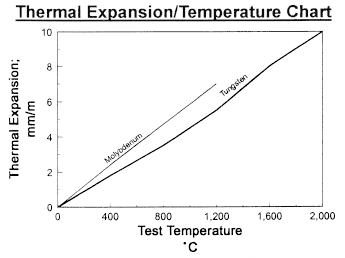
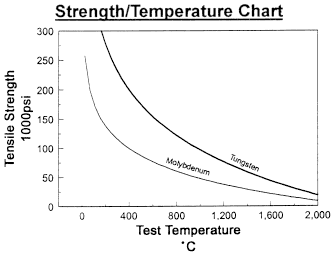
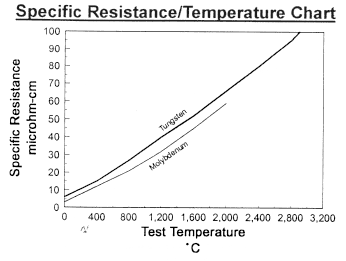
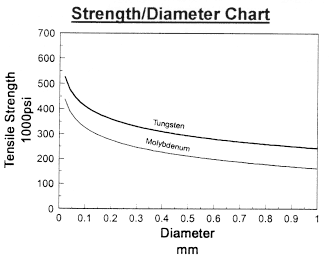
Chemical Reactivity of Molybdenum
R = Resistant. VR = Variable Resistance depending on temperature and concentration. NR = Non-resistant.
| Reagent | R | VR | NR |
|---|---|---|---|
| Water | X | ||
| Hydroflouric Acid1 | X | ||
| Hydrochloric Acid (cold) | X | ||
| Sulfuric Acid (hot) | X | ||
| Nitric Acid (cold) | X | ||
| Nitric Acid (hot) | X | ||
| Aqua Regia (cold) | X | ||
| Aqua Regia (hot) | X | ||
| Nitric/Hydroflouric mixture1 | X | ||
| Aqueous Ammonia | X | ||
| Aqueous Caustic Soda/Potash | X | ||
| Molten Caustic | X | ||
| Molten Caustics2 | X | ||
| Boron (hot)-Boride fomation | X | ||
| Carbon (1100°C)-Carbide Formation | X | ||
| Silicon (1000°C)-Silicide Formation | X | ||
| Phosphorous | X | ||
| Sulfide Formation (440°C) | X | ||
| Iodine | X | ||
| Bromine | X | ||
| Chlorine | X | ||
| Flourine (room temperature) | X | ||
| Oxygen or air (>400°C) | X | ||
| Oxygen or air (>600°C) | X |
| Reagent | R | VR | NR |
|---|---|---|---|
| Hydrogen | X | ||
| Nitrogen | X | ||
| Inert Gasses (all) | X | ||
| Carbon Monoxide (1400°C)-Carbide Formation | X | ||
| Carbon Dioxide (1200°C)-Oxidation | X | ||
| Hydrocarbons (1100°C)-Carbide Formation | X | ||
| Aluminum (molten) | X | ||
| Iron (molten) | X | ||
| Cobalt (molten) | X | ||
| Nickel (molten) | X | ||
| Tin (molten) | X | ||
| Zinc (molten) | X | ||
| Lead | X | ||
| Cesium | X | ||
| Gallium | X | ||
| Potassium | X | ||
| Lithium | X | ||
| Magnesium | X | ||
| Sodium | X | ||
| Mercury | X | ||
| Bismuth | X | ||
| KNO2, KNO3, KCLO3 (molten) | X | ||
| Molten Glass | X | ||
| Al2O3, BeO, MgO, ThO2, ZrO2(<1700°C) | X |
Notes: May be either hot or cold or Molten Caustics are in the presence of KNO2, KNO3, KCLO3, PbO2.
Chemical Reactivity of Tungsten
R = Resistant. VR = Variable Resistance depending on temperature and concentration. NR = Non-resistant.
| Reagent | R | VR | NR |
|---|---|---|---|
| Water | X | ||
| Water Vapor (red heat)-Oxidation | X | ||
| Hydroflouric Acid | X | ||
| Hydrochloric Acid | X | ||
| Sulfuric Acid | X | ||
| Nitric Acid | X | ||
| Aqua Regia (cold) | X | ||
| Aqua Regia (warm/hot) | X | ||
| Nitric/Hydroflouric mixture | X | ||
| Aqueous Caustic Soda/Potash | X | ||
| Ammonia | X | ||
| Ammonia in presence of H2O2 | X | ||
| Ammonia (<700°C) | X | ||
| Ammonia (>700°C) | X | ||
| Carbon (>1400°C)-Carbide Formation | X | ||
| Iodine (at red heat) | X | ||
| Bromine (at red heat) | X | ||
| Chlorine (>250°C) | X | ||
| Carbon Disulfide (red heat) | X | ||
| Mercury (and vapor) | X |
| Reagent | R | VR | NR |
|---|---|---|---|
| Flourine | X | ||
| Oxygen or air (<400°C) | X | ||
| Oxygen or air (>400°C) | X | ||
| In air | X | ||
| Hydrogen | X | ||
| Nitrogen | X | ||
| Carbon Monoxide (<800°C) | X | ||
| Carbon Monoxide (>800°C) | X | ||
| Carbon Dioxide (>1200°C)-Oxidation | X | ||
| Aluminum oxide-Oxidation | X | ||
| Magnesium Oxide-Oxidation | X | ||
| Thorium oxide (>2220°C)-Oxidation | X | ||
| Sodium Nitrite (molten) | X | ||
| Sulfur (molten, boiling) | X | ||
| Hydrogen/Chloride Gas (<600°C) | X | ||
| Nitric Oxide (hot)-Oxidation | X | ||
| Hydrogen Sulfide (red heat) | X | ||
| Sulfur Dioxide (red heat) | X | ||
| In presence of KNO2, KNO3, KCLO3, PbO2 | X |
Chemical Reactivity of Tantalum
R = Resistant. VR = Variable Resistance depending on temperature and concentration. NR = Non-resistant.
| Reagent | R | VR | NR |
|---|---|---|---|
| Acetic Acid | X | ||
| Acetic Anhydride | X | ||
| Aluminum Chloride | X | ||
| Aluminum Sulfate | X | ||
| Ammonia | X | ||
| Ammonium Chloride | X | ||
| Ammonium Hydroxide | X | ||
| Ammonium Nitrate | X | ||
| Ammonium Phosphate | X | ||
| Ammonium Sulfate | X | ||
| Amyl Acetate or Chloride | X | ||
| Aqua Regia | X | ||
| Arsenic Acid | X | ||
| Barium Hydroxide | X | ||
| Bromine, dry (<200°C) | X | ||
| Calcium Hydroxide | X | ||
| Calcium Hypochlorite | X | ||
| Chlorinated Brine | X | ||
| Chlor. Hydrocarbons | X | ||
| Chlorine, dry (<175°C) | X | ||
| Chlorine, wet | X | ||
| Chlorine Oxides | X | ||
| Chloracetic Acid | X | ||
| Chromic Acid | X | ||
| Chrome Plating Solutions | X | ||
| Cleaning Solution | X | ||
| Copper Salts | X | ||
| Ethylene Dibromide | X | ||
| Ethyl Chloride | X | ||
| Fatty Acids | X | ||
| Ferric Chloride | X | ||
| Ferric Sulfate | X | ||
| Ferrous Sulfate | X | ||
| Flourine | X | ||
| Formic Acid | X | ||
| Fuming Nitric Acid | X | ||
| Fuming Sulfuric Acid | X | ||
| Hydrobromic Acid | X | ||
| Hydrochloric Acid | X | ||
| Hydrocyanic Acid | X | ||
| Hydrofluoric Acid | X | ||
| Hydrogen Bromide | X | ||
| Hydrogen Chloride | X | ||
| Hydrogen Iodide | X | ||
| Hydrogen Peroxide | X | ||
| Hydrogen Sulfide | X | ||
| Hypochlorous Acid | X | ||
| Iodine (<1000°C) | X | ||
| Lactic Acid | X | ||
| Magnesium Chloride | X | ||
| Magnesium Sulfate | X | ||
| Mercuric Chloride | X |
| Reagent | R | VR | NR |
|---|---|---|---|
| Methyl Sulfuric Acid | X | ||
| Nickel Chloride | X | ||
| Nickel Sulfate | X | ||
| Nitric Acid | X | ||
| Nitric Acid, fuming | X | ||
| Nitric Oxides | X | ||
| Nitrous Acid | X | ||
| Nitrosyl Chloride | X | ||
| Organic Chloride | X | ||
| Oxalic Acid | X | ||
| Perchloric Acid | X | ||
| Phenol | X | ||
| Phosphoric Acid <4ppmF | X | ||
| Pickling Acids1 | X | ||
| Phthalic Anyhydride | X | ||
| Potassium Carbonate | X | ||
| Potassium Chloride | X | ||
| Potassium Dichromate | X | ||
| Potassium Hydroxide2 | X | ||
| Potassium Hydroxide3 | X | ||
| Potassium Iodide-Iodine | X | ||
| Silver Nitrate | X | ||
| Sodium Bisulfate, molten | X | ||
| Sodium Bisulfate, solution | X | ||
| Sodium Bromide | X | ||
| Sodium Carbonate | X | ||
| Sodium Chlorate | X | ||
| Sodium Chloride | X | ||
| Sodium Hydroxide2 | X | ||
| Sodium Hydroxide3 | X | ||
| Sodium Hypochlorite | X | ||
| Sodium Nitrate | X | ||
| Sodium Sulfate | X | ||
| Sodium Sulfide | X | ||
| Sodium Sulfite | X | ||
| Stannic Chloride | X | ||
| Sulfur (<500°C) | X | ||
| Sulfur Dioxide | X | ||
| Sulfur Trioxide | X | ||
| Sulfuric Acid (>160°C) | X | ||
| Zinc Chloride | X | ||
| Zinc Sulfate | X | ||
| Bismuth (<900°C) | X | ||
| Gallium (<450°C) | X | ||
| Lead (<1000°C) | X | ||
| Lithium (<1000°C) | X | ||
| Magnesium (<1150°C) | X | ||
| Mercury (<600°C) | X | ||
| Sodium (<1000°C) | X | ||
| Sodium - Potassium Alloys (<1000°C) | X | ||
| Zinc (<500°C) | X | ||
Comparative Machinability Ratings of Some Refractory Metals and Other Difficult Materials
Carbide Tool |
Machinability Ratings |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Workpiece Material | Hardness | Surface Speed (ft/min) | Cut Depth (in.) | Feed (in/rev) | Type | Life (in3) | Removal Rate (in3/min) | Relative Removal Rate | Relative Removal Cost |
Steel
|
|||||||||
| 4130 | 200 BHN | 445 | 0.12 | 0.019 | C6 | 582 | 11.50 | 100.0 | 1 |
| 4130 | 54RC | 90 | 0.12 | 0.004 | C6 | 19 | 0.62 | 5.4 | 19 |
Superalloys
|
|||||||||
| Rene 41 | 320 BHN | 70 | 0.06 | 0.009 | C2 | 23 | 0.47 | 4.1 | 25 |
| Rene 41 | 365 BHN | 70 | 0.06 | 0.009 | C2 | 16 | 0.47 | 4.1 | 25 |
Refractory Metals
|
|||||||||
| TZM | 217 BHN | 350 | 0.06 | 0.009 | C2 | 99 | 2.30 | 20.0 | 5 |
| Niobium | 112 BHN | 300 | 0.12 | 0.005 | C2 | 151 | 2.20 | 19.0 | 6 |
| Unalloyed, Wrought Molybdenum | 223 BHN | 275 | 0.10 | 0.010 | C1 | 132 | 3.30 | 29.0 | 4 |
Note:
Ratings based on metal removal rate for 4130 steel at 100,000 psi tensile strength as 100; lower numbers indicates poorer machinability.
Vacuum Furnaces
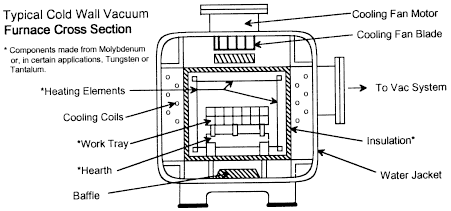
In the cold wall vacuum furnace design, heating is from within the vacuum vessel so the heat losses from the work area to the cold wall must be reduced. To be compatible with the vacuum system, the insulation must meet certain requirements. These include:
- low absorption
- absorption
- cleanliness; the material must not be prone to dusting which could be injurious to the vacuum pumps.
- low heat storage to facilitate cooling
- light weight
- high strength
Molybdenum is an ideal material for this application. Rembar stocks all the materials normally used in vacuum furnaces and can do much of the fabrication of both new and replacement parts. There are three basic insulation systems that will meet most of the above requirements. These systems can be classified as:
- Shield Pack
a series of non-contacting metal sheets. - Insulation Pack
an inner metallic shield supporting a blanket insulation against a metallic outer shell. - Self-facing Insulation
such as rigidized alumina-silica fibers and graphite felt.
The shield pack insulation system is composed of a multi-layer design that is made up of metal sheets that are separated to form a series of reflective shields. The selection of shield material is dependent on the maximum use-temperature of the system. Most commonly used is molybdenum.
The advantage of Radiant Molybdenum Shielding over other materials is:
- Cleanliness
Molybdenum will not flake off particles that could contaminate the work or pumping system. - Heat
Heat absorption of molybdenum is reflective to the radiant energy. This is the only way that heat can be transferred in a vacuum. - Outgassing
Molybdenum does not absorb gases as do the other materials. Therefore it does not outgas them during heat up to avoid prolonged pump down times. - Low Heat Storage
Molybdenum does not hold temperatures as long as the other materials and, therefore, allows for faster cooling.
Radiant Shield Data for Molybdenum
| Furnace Temperature, x F | 1832 | 1832 | 2012 | 2012 | 2400 | 2400 |
| Cold Shell Temperature, x F | 100 | 100 | 100 | 100 | 100 | 100 |
| Number of Shields (1 - 10) | 1 | 2 | 1 | 2 | 1 | 2 |
| Avg. Shield Emissivity Factor (0 - 1.0) | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 |
| Cold Shell Emissivity Factor (.9 typ) | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 |
| Computed Shield Temperature IN x F | ||||||
| #1 Shield | 1484 | 1646 | 1637 | 1811 | 1965 | 2167 |
| #2 Shield | 1250 | 1384 | 1672 | |||
| Computed Heat Loss (KW/ft2) | 4.0 | 2.4 | 5.5 | 3.3 | 9.8 | 5.9 |
| Furnace Temperature, x F | 2400 | 2400 | 2400 | 2400 | 2192 | 2192 |
| Cold Shell Temperature, x F | 150 | 150 | 150 | 150 | 100 | 100 |
| Number of Shields (1 - 10) | 3 | 4 | 5 | 6 | 1 | 2 |
| Avg. Shield Emissivity Factor (0 - 1.0) | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 |
| Cold Shell Emissivity Factor (.9 typ) | 0.70 | 0.70 | 0.70 | 0.70 | 0.90 | 0.90 |
| Computed Shield Temperature IN x F | ||||||
| #1 Shield | 2247 | 2282 | 2305 | 2320 | 1789 | 1976 |
| #2 Shield | 1976 | 2087 | 2151 | 2194 | 1517 | |
| #3 Shield | 1563 | 1832 | 1966 | 2047 | ||
| #4 Shield | 1444 | 1723 | 1869 | |||
| #5 Shield | 1354 | 1636 | ||||
| #6 Shield | 1282 | |||||
| Computed Heat Loss (KW/ft2) | 4.0 | 3.1 | 2.6 | 2.2 | 7.3 | 4.3 |
Machining and Welding Nickel-Iron Alloys
(ASTM F-15 and Similar Alloys)
In general, these alloys are not difficult to machine, provided it is noted that these materials work-harden readily. Also note that adequate care is taken on the choice of such factors as tool geometry and material, speeds, feeds, cutting fluids, etc. The following data is intended as a guide to proper selection of these parameters for machining Ni-Fe alloys.
General Machining
- Maximum rigidity of tool and workpiece must be obtained to insure smooth cutting.
- Machine speed should be kept low enough to provide sufficient torque or force at the cutting edge to prevent deceleration.
- Tools must be kept sharp with a high degree of surface finish on the rake face.
- Tool material should be either high speed steel or tungsten carbide.
Cutting Fluids
A large amount of heat is generated in cutting this material. Consequently, machining is made easier by using a good cutting fluid.
For general machine work, a copious flow (approximately 1 gpm/HP used) of soluble oil is recommended. A chlorinated oil is suggested for use on automatic and semi-automatic machines where a neat oil is required.
Turning and Boring
The general set up for these operations is similar to that used for steel. The following principle should be adhered to as closely as possible.
- It is preferable to take a deep cut with a light feed rather than a light cut with a heavy feed.
- Tool relief angles should be kept to a minimum in order to provide maximum support for the cutting edge.
- To insure adequate chip disposal when roughing, it may be necessary to vary the values for back or side rake angles slightly. This is to have the chip curl over and break on the workpiece in advance of the tool.
The following tool geometry, speed and feed values are given as a general guide for use with tungsten carbide tools. The speed and feed figures should generally be reduced by approximately 30% for high-speed steel tools.
| Detail | Roughing Value |
Finishing Value |
|---|---|---|
| Back rake angle | 8° | 10° |
| Side rake angle | 3° | 8° |
| Front cutting edge clearance angle |
3° | 3° |
| Slide cutting edge clearance angle |
3° | 3° |
| Plan trail angle | 8° | 5° |
| Plan approach angle* | 15° | 20° |
| Nose radius | 0.30 in (0.8 mm) |
0.05 in (1.3 mm) |
| Speed and Feeds |
Roughing | Finishing |
|---|---|---|
| Depth of cut | 0.1 in (2.5 mm) |
<=0.010 in (0.25 mm) |
| Feed (in-mm/rev) | 0.015 in (0.4 mm) |
>=0.004 in (0.10 mm) |
| Speed (SFPM) | 90 | 120 |
*Where it is impossible or impractical to apply this value, a decrease in plan approach angle should be followed by an increase in side rake and a decrease in back rake. Note that the secondary front cutting edge clearance angle should be to suit application.
Planing Techniques
To enable only a light cut to be taken with the finishing tool, roughing cuts should be taken to within approximately 0.25 in. (0.635 mm) of the finished dimension.
A goose neck type of planer tool is recommended for smoother finishing cuts since its shape enables it to withstand the greater mechanical shock encountered when machining Ni-Fe alloys.
The following tool angles are given as a general guide for use with high-speed tools.
| Detail | Roughing Value |
Finishing Value |
|---|---|---|
| Back rake angle | 8° | 10°-15° |
| Side rake angle | 15° | 8° |
| Front cutting edge clearange angle |
4° | 4° |
| Slide cutting edge clearance angle |
4° | 4° |
| Nose radius | 0.125 in (3 mm) |
0.250 in (6 mm) |
Drilling
The following principles should be observed when drilling Ni-Fe alloys:
- HSS twist drills with a high degree of flute finish should be used.
- Drills should be reground as soon as they show signs of dulling.
- A large amount of cutting fluid should flow onto the cutting edge of the drill.
- When hand feeding, sufficient pressure should be applied to keep the cutting edge beneath the work surface to prevent work-hardening the material.
- When drilling into a previously worked surface, it may be necessary to thin the drill web and increase the point angle from a nominal 118° to 150°.
- Peripheral speeds should be of the order of 50 surface feet per minute with feeds not less than 0.004 in/rev (0.1 mm/rev).
Precision Grinding
The methods for grinding Ni-Fe alloys are similar to those used with steel. However, certain conditions require that a slightly softer grade of wheel be used to prevent loading the wheel. A copious flow of lubricant should be used.
Where high permeability is required, final grinding (after annealing) should finish with approximately five cuts progressively decreasing from 0.002 in (0.05 mm) to 0.0002 in (0.005 mm).
Melting Points of Metals
| High | ||
|---|---|---|
| °C | °F | |
| Tungsten | 3410 | 6170 |
| Rhenium | 3180 | 5756 |
| Tantalum | 2996 | 5425 |
| Osmium | 2700 | 4892 |
| Molybdenum | 2610 | 4730 |
| Iridium | 2454 | 4449 |
| Ruthenium | 2450 | 4442 |
| Niobium | 2468 | 4379 |
| Boron | 2300 | 4172 |
| Hafnium | 2230 | 4046 |
| Medium | ||
|---|---|---|
| °C | °F | |
| Rhodium | 1966 | 3571 |
| Chromium | 1930 | 3506 |
| Zirconium | 1857 | 3375 |
| Thorium | 1845 | 3353 |
| Platinum | 1773 | 3223 |
| Titanium | 1725 | 3137 |
| Vanadium | 1710 | 3110 |
| Palladium | 1549 | 2820 |
| Iron | 1535 | 2795 |
| Cobalt | 1495 | 2723 |
| Yttrium | 1490 | 2714 |
| Nickel | 1455 | 2651 |
| Erbium | 1450 | 2642 |
| Beryllium | 1278 | 2332 |
| Manganese | 1220 | 2228 |
| Europium | 1150 | 2102 |
| Uranium | 1133 | 2071 |
| Copper | 1083 | 1981 |
| Samarium | 1072 | 1962 |
| Gold | 1063 | 1945 |
| Silicon | 1410 | 2570 |
| Low | ||
|---|---|---|
| °C | °F | |
| Neodymium | 1024 | 1875 |
| Silver | 961 | 1762 |
| Germanium | 947 | 1737 |
| Lanthanum | 920 | 1688 |
| Barium | 850 | 1562 |
| Calcium | 848 | 1558 |
| Cerium | 815 | 1499 |
| Arsenic | 814 | 1497 |
| Strontium | 774 | 1425 |
| Aluminum | 660 | 1220 |
| Magnesium | 651 | 1204 |
| Antimony | 630 | 1166 |
| Tellurium | 452 | 846 |
| Zinc | 419 | 786 |
| Lead | 327 | 621 |
| Cadmium | 321 | 610 |
| Thallium | 302 | 576 |
| Bismuth | 271 | 520 |
| Tin | 232 | 450 |
| Selenium | 217 | 423 |
| Lithium | 179 | 354 |
| Indium | 156 | 313 |
| Sodium | 98 | 208 |
| Potassium | 62 | 144 |
| Gallium | 30 | 8 |
| Mercury | -38.8 | -38 |
Densities of Metals
| High | |
|---|---|
| G/CC | |
| Osmium | 22.48 |
| Iridium | 22.42 |
| Platinum | 21.45 |
| Rhenium | 21.02 |
| Gold | 19.30 |
| Tungsten | 19.30 |
| Uranium | 19.05 |
| Tantalum | 16.60 |
| Mercury | 13.55 |
| Hafnium | 13.09 |
| Rhodium | 12.44 |
| Ruthenium | 12.20 |
| Palladium | 12.02 |
| Thallium | 11.85 |
| Thorium | 11.70 |
| Lead | 11.34 |
| Silver | 10.49 |
| Molybdenum | 10.20 |
| Medium | |
|---|---|
| G/CC | |
| Bismuth | 9.90 |
| Erbium | 9.16 |
| Copper | 8.96 |
| Cobalt | 8.92 |
| Nickel | 8.90 |
| Cadmium | 8.65 |
| Niobium | 8.57 |
| Iron | 7.87 |
| Manganese | 7.44 |
| Indium | 7.31 |
| Tin | 7.30 |
| Chromium | 7.14 |
| Zinc | 7.14 |
| Neodynium | 7.00 |
| Samarium | 6.93 |
| Cerium | 6.78 |
| Antimony | 6.68 |
| Zirconium | 6.50 |
| Tellurium | 6.24 |
| Lanthanum | 6.19 |
| Vanadium | 6.11 |
| Low | |
|---|---|
| G/CC | |
| Gallium | 5.97 |
| Arsenic | 5.73 |
| Germainium | 5.32 |
| Europium | 5.24 |
| Selenium | 4.81 |
| Titanium | 4.50 |
| Yttrium | 4.34 |
| Barium | 3.50 |
| Aluminum | 2.70 |
| Strontium | 2.60 |
| Boron | 2.34 |
| Silicon | 2.32 |
| Beryllium | 1.84 |
| Magnesium | 1.74 |
| Calcium | 1.55 |
| Sodium | 0.97 |
| Potassium | 0.86 |
| Lithium | 0.53 |
Brazing Filler Metals for Refractory Metals
| Brazing Filler Metal | Liquidus Temperature | |
|---|---|---|
| Ag | 1760°F | 960°C |
| Cu | 1980°F | 1052°C |
| Ni | 2650°F | 1454°C |
| Pd-Mo | 2860°F | 1571°C |
| Pt-Mo | 3225°F | 1774°C |
| Ag-Cu-Mo | 1435°F | 779°C |
| Ni-Cu | 2460°F | 1349°C |
| Mo-Ru | 3450°F | 1899°C |
| Pd-Cu | 2200°F | 1204°C |
| Au-Cu | 1625°F | 885°C |
| Au-Ni | 1740°F | 949°C |
American Wire Gauge No. (AWG) to Inch/MM
| Gauge No. | Inches | MM |
|---|---|---|
| 7/0 | 0.651300 | 16.54 |
| 6/0 | 0.580049 | 14.73 |
| 5/0 | 0.516549 | 13.12 |
| 4/0 | 0.460000 | 11.68 |
| 3/0 | 0.409642 | 10.40 |
| 2/0 | 0.364797 | 9.266 |
| 1/0 | 0.324861 | 8.251 |
| 1 | 0.289297 | 7.348 |
| 2 | 0.257626 | 6.544 |
| 3 | 0.229423 | 5.827 |
| 4 | 0.204307 | 5.189 |
| 5 | 0.181941 | 4.621 |
| 6 | 0.162023 | 4.115 |
| 7 | 0.144285 | 3.665 |
| 8 | 0.128490 | 3.264 |
| 9 | 0.114424 | 2.906 |
| 10 | 0.101897 | 2.588 |
| 11 | 0.090742 | 2.305 |
| 12 | 0.080808 | 2.053 |
| 13 | 0.071962 | 1.828 |
| 14 | 0.064084 | 1.628 |
| 15 | 0.057068 | 1.450 |
| 16 | 0.050821 | 1.291 |
| 17 | 0.045257 | 1.150 |
| 18 | 0.040303 | 1.024 |
| 19 | 0.035891 | 0.9116 |
| 20 | 0.031961 | 0.8118 |
| 21 | 0.028462 | 0.7229 |
| 22 | 0.025347 | 0.6438 |
| 23 | 0.022572 | 0.5733 |
| 24 | 0.020101 | 0.5106 |
| 25 | 0.017900 | 0.4547 |
| 26 | 0.015941 | 0.4049 |
| 27 | 0.014196 | 0.3606 |
| 28 | 0.012641 | 0.3211 |
| 29 | 0.011258 | 0.2860 |
| 30 | 0.010025 | 0.2546 |
| 31 | 0.008928 | 0.2268 |
| 32 | 0.007950 | 0.2019 |
| 33 | 0.007080 | 0.1798 |
| 34 | 0.006305 | 0.1601 |
| 35 | 0.005615 | 0.1426 |
| 36 | 0.005000 | 0.1270 |
| 37 | 0.004453 | 0.1131 |
| 38 | 0.003965 | 0.1007 |
| 39 | 0.003531 | 0.08969 |
| 40 | 0.003145 | 0.07988 |
| 41 | 0.002800 | 0.07112 |
| 42 | 0.002494 | 0.06335 |
| 43 | 0.002221 | 0.05641 |
| 44 | 0.001978 | 0.05024 |
| 45 | 0.001761 | 0.04473 |
| 46 | 0.001568 | 0.03983 |
| 47 | 0.001397 | 0.03548 |
| 48 | 0.001244 | 0.03160 |
| 49 | 0.001108 | 0.02814 |
| 50 | 0.000986 | 0.02504 |
| 51 | 0.000878 | 0.02230 |
| 52 | 0.000782 | 0.01986 |
| 53 | 0.000697 | 0.01770 |
| 54 | 0.000620 | 0.01575 |
| 55 | 0.000552 | 0.01402 |
| 56 | 0.000492 | 0.01250 |
| 57 | 0.000438 | 0.01113 |
| 58 | 0.000390 | 0.00991 |
| 59 | 0.000347 | 0.00881 |
| 60 | 0.000309 | 0.00785 |
