Tungsten Alloys Stock
Stock Supplies
These sizes are in stock, subject to prior sale. We also supply other sizes and alloys as well. See additional material information below.
sheet
Please call for availability
plate
Please call for availability
wire
Please call for availability
rod
Please call for availability
Material Information
- DFARS-Compliant Tungsten Alloy Available
- ASTM-B-777-15 Class 1,2,3,4
- ASTM Certifications upon request
- No cutting fee for custom lengths
- Custom Sizes Available
- Custom Stocking Plans Available
Helpful Links
Our Process
Tungsten Alloy Facts
Heavy Metal is made possible by P/M techniques. This is a technique where tungsten powder is mixed with nickel, iron or copper powder. It is then compacted and liquid phase sintered. The result is a very high-density machinable material having a homogeneous structure with no grain direction. This provides a material with unique physical properties and applications.
Technical Information
Tungsten Alloys
Because of the physical properties of heavy metal, it is often used as both a weight and structural member. Weights and counterbalances for aircraft control surfaces and rotor blades, guidance platforms, balancing of flywheels and crankshafts, vibration damping governors, and fuse masses and weights for self-winding watches are typical applications. Other specific applications include:
Radiation Shielding
Because of the absorption characteristics of heavy metals, approximately one-third less material is required as compared to lead. Heavy metal is used for source shielding on oil wells and industrial instrumentation as well as for collimators and shielding in medical therapy and detection equipment.
Rotating Inertia Members
Due to the material's unique combination of physical properties and high density, it can be rotated at extremely high speeds. This aspect makes it ideal for use in gyroscope rotors, flywheels, and rotating members for governors.
Projectiles
Properties such as elongation and hardness make heavy metal advantageous for use in kinetic energy penetrators. These properties can also be varied by manufacturing technique and additives. Heavy metal is used in squares, spheres, and projectile shapes for hypervelocity armor penetrating applications.
Boring Bars and Grinding Quills
Heavy metal is used for "chatter-free" boring and grinding. It is used where rigidity and minimum vibration are desirable. Heavier cuts and a better finish can be achieved with tools made of heavy metal. Longer tool extensions, with ratios up to 9:1 are also possible depending on the diameter of the tool.
Other benefits include:
Longer tool life due to the lower amount of heat generated as a result of the minimum chatter and high thermal conductivity that heavy metal provides. Heavy metals do not anneal during brazing, therefore carbide can be brazed directly with no effect on the shank. This allows continued use. Higher accuracy and trouble-free grinding are achieved when used as grinding quills. This is due to the vibration damping effect and rotating inertia characteristics of heavy metals.
Heavy metal is often used in place of Tungsten Carbide boring bars because:
- they have a higher density
- they are readily machinable
- they are less prone to chipping and breakage
- lower cost is achieved both with material and finishing
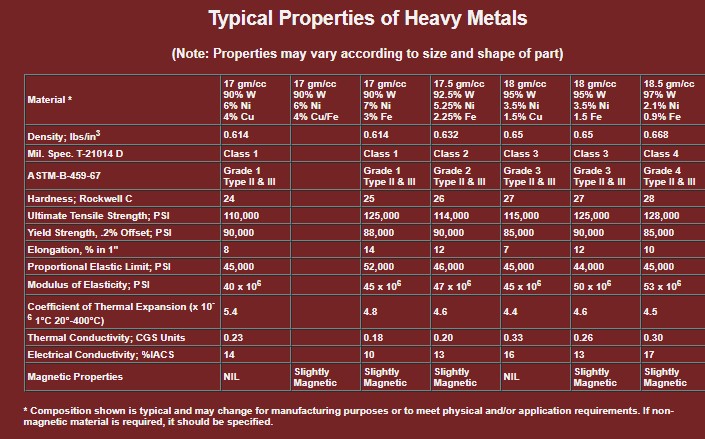
Typical Properties of Heavy Metals
Atomic Weight: 183.85
Density: 19.3 g/cc
Melting Point: 3695 K, 3422°C, 6192°F
Boiling Point: 6173 K, 5900°C, 10652°F
Mass:
Density at 20° C gm/cc - 19.3
Density at 20° C lb/in 3 - 0.697
Thermal Properties:
Melting Point, °C - 3410
Boiling Point, °C - 5900
Linear Coefficient of Expansion per °C - 4.9 x 10-6
Thermal Conductivity at 20°C, cal/cm2/cm°C/sec. - 0.40
Specific Heat, cal/g/°C; 20°C - 0.032
Electrical Properties:
Conductivity, % IACS - 31%
Resistivity, microhms-cm; 20°C - 5.5
Temperature Coefficient of Resistivity per °C (0-100°C) - 0.0046
Mechanical Properties:
Tensile Strength at room temperature, psi - 100,000-500,000
Tensile Strength-500°C psi - 75,000-200,000
Tensile Strength-1000°C psi - 50,000-75,000
Young's Modulus of Elasticity; lb/in2:
Room Temperature - 59 x 106
500°C - 55 x 106
1000°C - 50 x 106
Spectral Emissivity:
(Wave Length approx. 0.65) - 0.37 (900°C)
Working Temperature: 1700°C
Recrystallizing Temp: 1200-1400°C
Stress Relieving Temp: 1100°C
Metallography:
Etchant - HF-NH; F sol
Polishing - Alumina - Rouge to finish
